General Aspects Agglomeration and Agglomerator Systems
Numerous food powders experience significant changes in their properties during storage, transportation, or processing, which are related to the particle size distribution. Attrition causes reduction in average particle size while aggregation increases it. Fines generated by attrition may either form clusters or coat larger particles (plating). Interparticle adhesion is decisively influenced by particle size, the ratio between adhesion and weight usually being inversely proportional to the square of the particle size.
As a result, this ratio is two orders of magnitude higher for particles of 10µm than for particles of 100µm. Dry food powders with average sizes of 80 to 100 µm are usually free flowing, whereas powders having sizes below 20 to 30µm become cohesive, form secondary particles (clusters) of larger size, and form lumps when rewetted.
Primarily because of the differences in particle size and also in density, shape, and resilience, fine particles migrate to the bottom while large particles find themselves at the top of the vessel. As a result, some minor components of beverage blends (colors, flavors, vitamins) may become unevenly distributed between packages. The purpose of particle size enlargement by agglomeration is to improve powder properties like bulk density, flowability, meterability, dusting, powder mix homogeneity, storage stability, and optical appearance. Powdered foods, which are in most cases intended to be dispersed in liquid, should also have good wettability, sinkability, dispersibility, and (for soluble materials) solubility, that is good "instant properties."
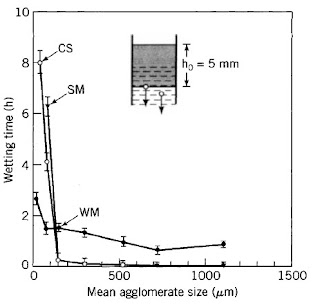
A powder layer spread on a liquid surface should imbibe the liquid, submerge, disperse, and dissolve within a few seconds with little mechanical aid and without forming lumps. A powder treated by a technical process to have such properties is called "instantized." Agglomeration is the predominant method for instantizing powdered foods, and an example of the dependency of the wetting time of a powder layer on the average agglomerate size is shown in Figure 1.
Another major quality factor for instant foods is the preservation of flavor components. Instant beverages containing, for example, coffee extract are particularly susceptible to flavor loss caused by high temperature or excessive contact with air, as, for example, in a fluidized bed. Obviously, simultaneous improvement of all powder properties is impossible.
Increasing agglomerate stability, for example, results in most cases in a decline of the instant properties. The way the agglomerates are formed in the production process determines their properties, and comprehension of the basic physical principles of particle adhesion and the mechanisms likely to predominate in a given agglomeration process is helpful.
UNDESIRED AGGLOMERATION - CAKING
Agglomeration via caking may occur unintentionally since blends of particles are always exposed for some time to the ambient environmental conditions (temperature and/or humidity). For example, food powders that include lipids (soups, sauces, baking mixes) may undergo caking if the temperature exceeds the melting point of the lipids.
As a result, sticky liquid bridges are formed. Once cooled, the lipids recrystallize, liquid bridges between particles become solid, and caking is reinforced. Although starchy and proteinaceous components are relatively insensitive to the environmental conditions, the soluble components of food powders (sugars, salts) absorb moisture and eventually change their state from solid to liquid.
The ability of sugars to soften depends on the conditions under which they were produced and stored. These conditions are responsible for the formation of areas of crystalline or amorphous structure. Amorphous sugars absorb much more moisture at a given water activity (relative humidity) and have lower glass-transition temperatures than crystalline sugars. Whereas a stable, crystalline structure is formed at equilibrium conditions, the amorphous one is created at nonequilibrium conditions.
Relatively slow moisture withdrawal during carefully controlled crystallization (nuclei formation and crystal growth) leads to the development of a crystalline structure. Fast moisture withdrawal from a solution of carbohydrate via spray drying, roller drying, or freeze drying helps to produce mainly the amorphous form; even the mechanical impact of milling of sugar crystals produces an amorphous surface capable of recrystallization after absorbing water.
Upon recrystallization, amorphous sucrose releases water, which facilitates formation of bridges between particles and initiates caking. Adding high molecular weight components (eg, maltodextrin) to a blend containing sugars may reduce caking. Caking may be effectively suppressed by adding anticaking agents like tricalcium phosphate, magnesium oxide, calcium silicate, and so on, which absorb a portion of moisture from the blend and thus reduce the amount of available moisture.
Although total moisture content of the blend with or without anticaking agent stays virtually unchanged, it is relative humidity generated by the blend in a sealed chamber that reflects the amount of available moisture: blend with added anticaking agent generates lower RH than blend without anticaking agent. The effectiveness of the anticaking agents depends largely on their water-holding capacity, so that with an unlimited source of humidity (open storage), their impact is lessened. Even packaged food powders may undergo caking influenced by the environment inside their packages.
Being relatively isolated, the headspace inside the package is affected not only by the surface moisture of the particles and temperature in the warehouse, but by the permeability and heat conductivity of the package film. Variations in the temperature and humidity outside of the packaged material often accelerate an exchange in surface moisture between the ingredients and initiate caking.
AGGLOMERATION METHODS
The first step in any agglomeration process (except drying methods starting with a slurry) is to make the primary particles contact each other, which is frequently achieved by external force. Powders for instant products usually consist of primary particles smaller than 200 µm to facilitate solubility. In a second step, permanent adhesion forces stronger than any possibly existing disruptive forces must be established between these particles. For food powders, this is usually achieved by wetting (which causes partial dissolution and the development of liquid bridges) and subsequent drying (which leaves solid bonds in place of the liquid bridges).
The duration and intensity of the forces acting among the particles during agglomerate formation and stabilization have an important influence on agglomerate porosity and stability. For example, an agglomeration process in which high forces act on the particles and agglomerates will turn out dense, smooth, and stable agglomerates that are easy to handle and dispense. However, instant properties would be poor owing to low agglomerate porosity and strong bonds between the primary particles. Such a process, like compaction, would be inappropriate for instantizing. The final product should have the following properties:
- sufficient agglomerate porosity for fast liquid suction by capillary action, although a critical porosity must not be exceeded;
- particle size in the range of 0.2 to 2 mm; and
- sufficient agglomerate strength to withstand handling and transportation.
Agglomeration processes suitable for producing instantized food powders can be divided into three groups:
- moist agglomeration,
- agglomeration by drying, and
- combined methods.
Moist Agglomeration
Moist agglomeration, using capillary and liquid bridge forces to achieve sufficient interparticle adhesion during agglomeration, is the most important process for the production of instantized powders. This method starts from dry powder, which is moistened either by condensing vapor, atomized liquid, or a mixture of both.
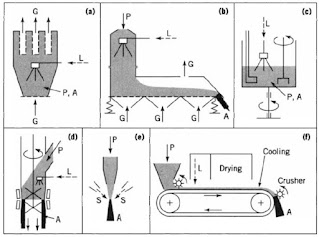
The material is then dried and solid bridges between the primary particles provide the necessary strength. A large variety of equipment is available for moist agglomeration. Schematics of some typical examples are shown in Figure 2. Except for the static process Figure 2f, all methods are "dynamic"; that is, agglomerates are formed due to the collision and subsequent adhesion of the particles.
Common features of all processes shown in Figure 2 are the moistening of the dry powder, the size enlargement of the wet particles, and subsequent drying and cooling, if required. The drying rate and temperature considerably influence the strength of the dry agglomerates because of different crystal structures and distributions of the interparticle solid bridges formed by crystallization of dissolved substances during drying.
Fluidized-bed agglomerators for batchwise or continuous production, Figure 2a and 2b, are provided by numerous manufacturers. Units for continuous operation (eg, APV Anhydro) utilize either a moisture product feed or a rewet system so that sufficient moisture is present to agglomerate the product.
A typical unit has three fluidized zones. These include the entry or wetting zone, a drying zone, and a cooling zone. Also, several mechanical agglomerators utilize mechanical mixers to provide the liquid addition, product interaction, and mixing to facilitate the agglomeration process.
The "SCHUGI" mixer (Bepex Corp.), shown in Figure 2d, has extremely short residence times (approximately 1 s) and has considerable flexibility in the types and amounts of feedstock. The system employs a flexible housing so that product buildup on the interior walls of the agglomerator is minimized, if not eliminated.
Jet agglomeration (Fig. 2e) has been used in the food industry for several years to produce agglomerates with favorable instant properties from fine powders. In a jet agglomeration plant, freely moving, wetted particles are made to collide with each other to form agglomerates.
The solid material fed to the agglomerator consists of individual particles and dry preagglomerates bound mainly by van der Waals forces. The effectiveness of this method depends on a variety of process parameters influencing interparticle collision frequency, relative velocities of the particles, interparticle contact forces between wetted particles, and strain on the agglomerates.
Drying Processes
The second main group of processes for agglomerating food powders comprises special drying processes. Two examples are shown schematically in Figure 3, a spray drier with fluidized bed and a freeze drier.
Spray drying is one of the most widely used processes in food powder technology. The concentrate/slurry, either in the form of a suspension or a solution, is finely distributed using a nozzle or an atomizer disc, dried, and cooled in a connected fluidized bed from which the product is withdrawn in agglomerated form.
The fluidized bed also serves the purpose of removing the fines, which are collected in a cyclone and recycled into the spray drier. Agglomeration occurs in the vicinity of the nozzle or atomizer, where the fine dry particles collide with the slurry droplets.
In many cases the product is also after-dried in the fluidized bed, a process referred to as two-stage drying. Freeze drying is relatively expensive but especially useful for products sensitive to high temperatures. Another advantage is the possibility to vary the porosity of the agglomerates over a wide range by foaming the concentrate before freezing (this can be achieved in a spray drier, too, by gassing the slurry immediately before atomization). As a novel technology, microwave drying in a vacuum chamber can be used instead of freeze drying.
Combined Methods
Two examples for combined agglomeration methods are presented in Figure 4. These are special spray driers, the "Filtermat" by GEA Niro AIs (Soeborg, Denmark), with integrated perforated belt drier (Fig. 4a), and spray drying with integrated fluidized bed and fines recycling (Fig. 4b). In the "Filtermat" process, the product is partially dried in the spray-dry section of the machine to a moisture content of approximately 16 to 25%, whereupon it is deposited by gravity onto the perforated belt and further dried to approximately 5 to 10% moisture.
Sufficient moisture is present in the intermediate dried product so that agglomerates are formed on the belt. These are then classified and/or size-reduced to the desired size. These methods allow production of very loose, but still sufficiently strong agglomerates with good instant properties and have been widely used in the food industry.
Further Methods Pressure agglomeration is rarely applied to the production of redispersible products, because the resulting agglomerates are so compact that they show insufficient dispersibility. Substances dispersing readily—due to a bursting effect of special additives—can be agglomerated using die pressing or low pressure extrusion processes.
If no great demands are set upon the redispersibility, roll pressing is a useful and inexpensive process. Mostly low-pressure ring-roller presses are used in which the moistened powder is pressed through holes and thereby shaped into agglomerates. A special kind of agglomeration is coating, to improve the wetting behavior of the particles. Usually the particles are coated by pure liquids, solutions, or suspensions that harden on the surface of the solid material. A novel technology is coating with submicron particles by "mechanofusion" to improve the wettability and for other applications.
 |
| Figure 3. Principles of production of instantized powdered foods by spray drying (a) and freeze drying (b). A, agglomerates; C, concentrate; F, fines; G, gas |
CHARACTERIZATION OF AGGLOMERATED POWDERS
Particle size distribution of agglomerated products should be measured using an adequately representative sample and with minimum disruption. Care should be taken to prevent swelling, dissolution, or disintegration. For sufficiently stable particles, sieving is still widely in use (dry for particles larger than 30 to 40 µm and wet for smaller particles), but over the past few years, laser diffraction has become a standard method due to the availability of a large number of different devices (Coulter, Malvern, Sympatec, etc).
The last method is more versatile because it allows the measurement of larger particles with a dry feeder in a relatively nonviolent way and is also applicable for fines in a dispersing liquid. Automatic image analysis systems are a recent development employing charge-coupled device (CCD) camera imaging of particles falling out of a dry feeder. Agglomerated particles may partially disintegrate when subjected to a more or less violent impact (vibration, shaking) during storage, transportation, and handling.
In the case of agglomerated coffee, attrition is the primary cause in separation of fines from the outer surface of agglomerates. Formation of so-called secondary particles (shattering) from the disintegrated pieces is also taking place. The strength or resistance of agglomerated products to attrition may be measured by comparing its size distribution before and after rotation for a limited time inside a cylinder (friabilator).
Other properties of agglomerates (bulk or free flow density, flowability, cohesion, angles of spatula and repose) are measured, for example, with the Hosokawa (MicroPul) tester. For powdered foods, information about wetting and dispersing behavior is of special interest to the manufacturer. Wetting of a powder is easily tested by preparing a sample of defined height (5-8 mm) in a cylindrical testing vessel with a slide covering the liquid reservoir.
When the slide is pulled out sideways, the powder sits on the liquid surface and is wetted. The wetting time measured in this way is useful for quality control purposes and for product property comparison.
Powder dispersion measurement is more difficult and depends on the ability of measuring the amount of material actually dispersed after the mixture/dispersion has been prepared in a defined way. For many products, this can be achieved by, for example, photometry (milk powder, cocoa beverages), conductometry (powdered extracts of coffee, tea) or refraction index measurement (sugars). The dispersed powder mass divided by the total powder mass in the sample is called degree of dispersibility (which varies between 0 and 1). Milk powder can reach a value of up to 0.8 (depending on fat content and age), instant cocoa beverages up to 0.98 and instant coffee up to 1.
PRODUCT EXAMPLES
Agglomerated food products are inseparable from some specific processes that were used to obtain these products. Steam fusion/steam jet agglomeration is used for agglomerating water-soluble instant beverage powders with high sugar content like cocoa drinks or products containing coffee extract (Fig. 5). After grinding, the particles with an average particle size of 25 to 75 µm are fed into an agglomeration chamber in the shape of dry clusters (average size up to the mm range). A uniformly distributed curtain of powder moves downward where it interacts with jets of steam.
Steam wets the particles and fuses them into agglomerates. The wetted agglomerates pass from the top section of the agglomerating tower into a drying zone in the bottom portion of the tower, which is supplied with hot air. The agglomerates are dried and then cooled and screened. Critical parts of this agglomeration process are the feed port section, where initial cluster formation occurs, and the steam jet zone, where further agglomeration by collision of sticky clusters takes place.
A method for making agglomerated bits containing aspartame includes preblending aspartame and a bulking agent (maltodextrin) to form a premix. The latter is then mixed with other dry ingredients (flavors, starch binders, dispersing agents, and vitamins) to form a dry mix. Liquid ingredients (vegetable oil and water) are blended into a dry mix with a ribbon blender or a paddle mixer to form moistened clumps. These granules must be dried in a forced-air convection oven and then screened to obtain a desirable particle size distribution.
While starches (tapioca, corn, potato, modified wheat) and gums are used as the binders in forming agglomerates, baking soda and maltodextrin assist in the dispersion of the agglomerated product. These bits are suitable for use in home-cooked grain cereals and other foods. Agglomerated potato granules can be prepared from a mixture of potato granules, egg white solids, and water. After the wet premix is formed, a gentle sieving, drying, and crushing are used to obtain agglomerates with the desired size and density.
Agglomerated bread crumbs may be produced from a starch containing raw material (flour, meal) and water in a continuous pellet mixer; subsequent baking in a humidified atmosphere (to control the desired gelatinization) and sizing (cutting) of the agglomerates are utilized. A controlled retrogradation (recrystallization of starch) occurs due to the controlled cooling process. Agglomerated beverage blends having aspartame as a sweetener may be manufactured to prevent clumping of aspartame and to improve its water dispersibility.
Agglomeration has been conducted in a jacketed blender so that a heating or cooling fluid may be passed through the jacket while the blender is rotated to provide adequate mixing. The blending time and temperature (but not, however, humidity) were controlled to obtain a desired agglomerate size distribution. Aqueous, sugary syrups (honey, high fructose corn syrup, invert sugar, corn syrup, etc) were dehydrated using thin film drying in the presence of binders (soy protein and ungelatinized starch that was partially gelatinized in situ).
A spray of water was added during tumbling. The resultant agglomerates were dried and then slightly coated with a high melting point fat, apparently to prevent caking. Agglomerates of garlic, onion, and their mixtures were produced in an upright chamber. Wetting of particles was provided by atomized water in the upper part of this chamber, followed by drying of the agglomerated product with air in the lower part of the chamber.
An agglomerated milk product was prepared by spraying a concentrate of milk into a stream of drying gas directed against the surface of a fluidized layer of already spray-dried particles. Adjustment of temperatures, flow rates of drying air, and residence times allowed better efficiency for skim milk, whole milk, and whey particles.
 |
| Figure 5. Principle flow diagram of a steam fusion/steam jet agglomeration process. |
Agglomerates of meat analogues were prepared by extrusion cooking of soy concentrate, comminution of the extrudate, mixing it with a water slurry of binder, frying the mixture in edible fat or oil to produce an agglomerated mat, and sizing. Particles with the desired size distribution were used for meat-type sauces.
A porous and pelletized food product may be formed by premixing two or more ingredients, one of which is capable of forming sticky bonds after being moistened by an aqueous medium. This interaction occurs when the mixture of particles is tumbled and rolled on a pelletizing disc; the adhered particles form pellets or wet aggregates.
Examples of a dry mix may include sugars, starches, dried milk products, proteinaceous materials, dehydrated juices, and powdered coffee concentrates. Some of these products (sugars, starches) may become self-adherent in contact with water and are used to form agglomerates. To provide pellets with controlled porosity, the particulate mixture also includes a chemical leavening system (sodium bicarbonate and leavening acid).
Once moist agglomerates are in contact with hot air, two processes take place: drying and formation of gaseous carbon dioxide (due to a reaction between the bicarbonate and leavening acid). The resulting pellets have a porous, cellular structure with a crisp, crunchy, and friable texture. The vast majority of gelatin dessert mixes require the use of hot water to dissolve the gelatin and an extended time (3-4 h) to prepare the meal. However, if gelatincontaining mix with a limited moisture content of 1 to 3% is agitated and slowly heated to 190 to 195°F, it forms agglomerates.
Subsequent cooling helps to form so-called cold-water-soluble gelatin, which dissolves and disperses in water at 40 to 50°F. Agglomeration of gelatin mixes (ie, sucrose, gelatin, citric acid) was conducted in a jacketed rotating blender. Other agglomerated (instantized) products include maltodextrin and dextrose, which may be used as carriers for flavors, colors, and nonnutritive sweeteners in instant beverages and desserts, soy protein isolates for highprotein beverage blends and for better dispersibility in meat emulsions, prejelled starches and gums as soup thickeners, whey protein concentrates and calcium casemates for dairy blends, all developed by IFT, Inc., and coated animal feed with improved nutritional value. Powders such as egg proteins, cocoa, or various fibers will have improved dispersibility after agglomeration in the presence of maltodextrin or surfactants.