Alkaloids: Classification And Properties
Alkaloids are naturally occurring substances with a particularly wide range of structures and pharmacologic activities. They may be conveniently divided into three main categories: the true alkaloids, the protoalkaloids, and the pseudoalkaloids.
The true alkaloids have the following characteristics: they show a wide range of physiological activity, are usually basic, normally contain nitrogen in a heterocyclic ring, are biosynthesized from amino acids, are of limited taxonomic distribution, and occur in the plant as the salt of an organic acid. Exceptions are colchicine, aristolichic acid, and the quaternary alkaloids. The protoalkaloids, also known as the biological amines, include mescaline and N,N-dimethyltryptamine.
They are simple amines synthesized from amino acids in which the nitrogen is not in a heterocyclic ring. The pseudoalkaloids, those not derived from amino acids, include two major series of compounds: the steroidal and terpenoid alkaloids (eg, cones-sine) and the purines (eg, caffeine). Most alkaloids occur in the Angiosperms, the flowering plants, but they are also found in animals, insects, marine organisms, microorganisms, and the lower plants.
Physical Properties
Most alkaloids are colorless crystalline solids with a defined melting point or decomposition range (eg, vindoline and morphine). Some alkaloids are amorphous gums and some are liquids (eg, nicotine and conine) and some are colored (eg, berberine is yellow and betanidine is red). The free base of the alkaloid is normally soluble in an organic solvent; however, the quaternary bases are only water soluble, and some of the pseudo- and protoalkaloids show substantial solubility in water. The salts of most alkaloids are soluble in water. The solubility of alkaloids and their salts is of considerable significance in the pharmaceutical industry, both in the extraction of the alkaloid from plant or fungus and in the formulation of the final pharmaceutical preparation. Solubility is also of considerable significance in the clinical distribution of an alkaloidal drug.
Chemical Properties
Most alkaloids are basic, which makes them extremely susceptible to decomposition, particularly by heat and light.
Ornithine-Derived Alkaloids
Ornithine-derived alkaloids include the tropanes (atropine, l-hyoscyamine, l-scopolamine, and cocaine), the Senecio alkaloids, and nicotine. Tropane. Tropane alkaloids are derived from plants in the Solanaceae, Erythroxylaceae, and Convolvulaceae families. These alkaloids comprise two parts: an organic acid and an alcohol (normally a tropan-3α-ol). The pharmacologically active members of this group include atropine, the optically inactive form of Z-hyoscyamine, which is isolated from deadly nightshade (Atropa belladonna), l-hyoscyamine and l-scopolamine, which are found in the leaves of Duboisia metel L., D. meteloides L., and D. fastuosa var. alba.
The tropane alkaloids are parasympathetic inhibitors. For example, atropine acts through antagonism of muscarinic receptors, the receptors responsible for the slowing of the heart, constriction of the eye pupil, vasodilation, and stimulation of secretions. Atropine prevents secretions (eg, sweat, saliva, tears, and pancreas) and dilates the pupil. Atropine is used to reduce pain of renal and intestinal cholic and other gastrointestinal tract disorders, to prolong mydriasis when necessary, and as an antidote to poisoning by cholinesterase inhibitors.
Small doses produce respiratory and myocardial stimulation and decrease nasal secretion, and the drug has little local anesthetic action. Hyoscyamine and scopolamine have mydriatic effects. They are also used in combination as sedatives, in anti-motionsickness drugs, and in antiperspirant preparations.
Cocaine. Cocaine is a potent central nervous system (CNS) stimulant and adrenergic blocking agent. It is extracted from South American cocoa leaves or prepared by converting ester alkaloids to exgonine, followed by methylation and benzoylation. It is too toxic to be used as an anesthetic by injection, but the hydrochloride is used as a topical anesthetic. It has served as a model for a tremendous synthetic effort to produce an anesthetic of increased stability and reduced toxicity.
 |
| Cocaine |
Senecio Alkaloids. Senecio alkaloids possess a pyrrolizidine nucleus and occur in the genera Senecio (Compositae), Heliotropium and Trichodesma, and Crotalaria. They are biosynthetically derived from two units of ornithine in a manner similar to some of the lupin alkaloids. Certain of the alkaloids having an unsaturated nucleus are potent hepatotoxins.
Nicotine. Nicotine is toxic, soluble in water, and a constituent of tobacco. The lethal human dose is ca 40 mg/kg. Pharmacologically, there is an initial stimulation followed by depression and paralysis of the autonomic ganglia. The biosynthesis of nicotine is well established.
 |
| Nicotine |
Lysine-Derived Alkaloids
Lysine-derived alkaloids contain the pyridine nucleus or its reduced form, piperidine. They include alkaloids derived from the Areca or Betel nut, Lobelia alkaloids, and those derived from pomegranate or club mosses. Arecoline, a colorless liquid alkaloid that has a pronounced stimulant action (in large doses, paralysis may occur) is found in the Areca or Betal nut.
As the hydrobromide it is used as a diaphoretic and anthelmintic. Lobelia inflata, known as Indian tobacco, contains lobeline, which is similar to, but less potent than, nicotine in pharmacologic action and is used as an emetic; the sulfate salt is used in antismoking tablets. The root ofPunica granatum contains alkaloids such as pelletierine and pseudopelletierine, which are formed from lysine and acetate.
Pelletierine, is toxic to tapeworms and is used as an anthelmintic. The club mosses, Lycopodium spp., produce polycyclic alkaloids such as lycopodine, whereas Hydrangea spp. yield febrifugine, an active antimalarial agent. Anabasine is found in Haloxylon persicum Bunge; this alkaloid has antismoking and respiratory muscle stimulation action similar to lobeline and is also used as a metal anticorrosive.
A host of complex alkaloids such as lupinine, sparteine, cytisine, and matrine are found in the lupins, a large plant family of the Leguminosae. Sparteine paralyzes motor nerve endings and sympathetic ganglia. The sulfate is used as an oxytoxic and the adenylate derivative is used to treat cardiac insufficiencies. Cytisine, found in the seeds of the highly toxic plant Cytisus laburnum L., is a strongly basic alkaloid that produces convulsions and death by respiratory failure.
 |
| Pelletierine and Lupinine |
Anthranilic Acid-Derived Alkaloids
Anthranilic acid-derived alkaloids exhibit great structural diversity. This group includes dictamnine, platydesmine, vasicine, cusparine, and rutecarpine. Vasicine has oxytocic activity.
Phenylalanine- and Tyrosine-Derived Alkaloids
Phenylalanine- and tyrosine-derived alkaloids are by far the most numerous group of alkaloids, ranging from simple phenethylamines to the very complex dimeric benzylisoquinolines and the highly rearranged Cephalotaxus alkaloids.
Ephedrine. Ephedrine, from the Chinese drug Ma Huang, is soluble in water, alcohol, chloroform, and diethyl ether. It melts over the range of 33-42°C, depending on the water content. Little of the ephedrine of United States commerce is obtained from natural sources. Ephedrine is produced commercially through a biosynthetic process. Ephedrine has mydriatric effects and demonstrates adrenaline-like activity. It causes a rise in arterial blood pressure, increased secretions, and dilated pupils; it is a monoamine oxidase inhibitor and is used in nasal decongestants.
Peyote. Peyote, the small cactus of the Indians of north central Mexico, contain over 60 constituents. It is used as a hallucinogen in religious ceremonies and for medicinal purposes. A principal constituent is mescaline, a simple trime thoxy phene thylamine.
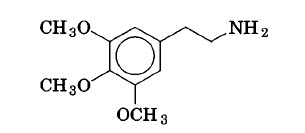 |
| Mescaline |
Ipecac. Ipecac, derived from Cephaelis ipecacuanha (native to Brazil), contains emetine and cephaeline, both of commercial significance. Emetine exhibits profound pharmacologic effects including clinical antiviral activity and is used in the treatment of amebic dysentery. Its side effects are cardiotoxicity, muscle weakness, and gastrointestinal problems including diarrhea, nausea, and vomiting. Two synthetic routes to emetine are of commercial importance: the Roche synthesis, which produces dehydroemetine, and the Burroughs-Wellcome synthesis. In handling emetine and its products, exposure should be limited as it can cause severe conjunctivitis, epidermal inflammation, and asthma attacks in susceptible individuals.
Isoquinoline and Related Alkaloids
Isoquinoline and related alkaloids are the largest group of alkaloids derived from phenylalanine (or its hydroxylated derivatives) and corresponding β-phenylacetaldehydes. The alkaloids are prevalent in the plant families Fumariaceae, Papaveraceae, Ranunculaceae, Rutaceae, and Berberidaceae.
There are many structural types, including the simple tetrahydroisoquinolines; the benzylisoquinolines; the bisbenzylisoquinolines, such as the dl- and dl-isomers of tetrandrine, which exhibit anticancer activity; the proaporphines, such as glaziovine, which is an antidepressant; the aporphines, such as glaucine; the aporphinebenzylisoquinoline dimers, such as thalicarpine, which shows cytotoxic and antitumor activity; the oxoaporphines; the protoberberines, a group of over 70 alkaloids such as xylopinine, berberine, canadine, and corydaline, known for such diverse pharmaceutical uses as tranquilizers, CNS depressants, antibacterial and antiprotozoal agents, anticancer agents, and alpha adrenergic blockers; the benzophenanthridines, a group of 30 alkaloids such as fagaronine and nitidine, which exhibit antitumor properties; the protopines; the phthalideisoquinolines, a group that includes narcotine, which possesses antitussive activity, depresses smooth muscles, and is not narcotic, and hydrastine, which is used as an astringent in mucous membrane inflammation; and the homoaporphines.
The Opium Alkaloids
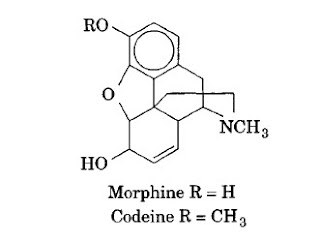
The opium alkaloids number over 25, some of which are of commercial importance and major significance. Opium is the air-dried milky exudate from incised, unripe capsules of Papaver somniferum L. or P albumen Mill (Papaveraceae). Notable opium-derived alkaloids include morphine, codeine, thebaine, noscapine, and papaverine.
Morphine is the most important alkaloid. It is isolated from opium. Along with its salts, it is classified as a narcotic analgesic and is strongly hypnotic. Side effects include constipation, nausea, and vomiting in addition to habituation, reduced power of concentration, and reduction in fear and anxiety. Respiration is also deepened. Codeine is the methyl ether of morphine, and thebaine is one of the methyl enol ethers of codeinone.
Codeine pharmacologically resembles morphine but is weaker, less toxic, and exhibits less depressant action (it does not depress respiration in normal therapy). It is used in the treatment of minor pain and as an antitussive. Codeine is available as a free base or the sulfate or phosphate salt. Thebaine is a convulsant poison rather than a narcotic.
Amaryllidaceae
Amaryllidaceae alkaloids include galanthamine, margetine, and narciprimine. Galanthamine is a water-insoluble crystalline alkaloid that exhibits powerful cholinergic activity and analgesic activity comparable to morphine. It has been used to treat diseases of the nervous system. Its derivatives show anticholinesterase, antibacterial, and CNS depressant activity. Narciclasine, margetine, and narciprimine exhibit anticancer activity.
Colchicine
Colchicine, also known as hermodactyl, surinjan, and ephemeron, has some of the most unusual solubility characteristics of any alkaloid: it is soluble in water, alcohol, and chloroform, but only slightly soluble in ether or petroleum ether. Colchicine-type alkaloids are present in ten other genera of the Liliaceae and 19 species of Colchicum. Reviews of the chemistry of colchicine and related compounds and their history and pharmacology are available. Colchicine has the ability to artificially induce polyploidy or multiple chromosome groups. It is also used to suppress gout.
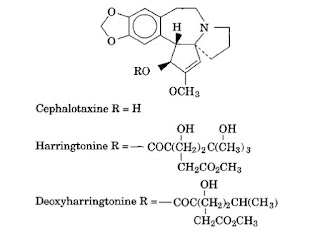
Cephalotaxus
Securinine
Securinine, isolated from Securinega suffruticosa Rehd, is similar to strychnine in action, but exhibits lower toxicity, stimulates respiration, raises blood pressure, and increases cardiac output. The chemistry, pharmacology, and biosynthesis of securinine and related compounds have been reviewed.
Tryptophan-Derived Alkaloids
Tryptophan-derived alkaloids, which occur in the families Apocynaceae, Rubiaceae, and Loganiaceae, have recently become of great interest, particularly those derived from tryptamine and a monoterpene unit. There are many diverse structural types in this group. Attempts to determine the important details of the biosynthetic interconversions of these compounds have been reported. The simplest indole alkaloids are derived from tryptamine itself.
These include indole-3-acetic acid, a potent plant-growth stimulator; serotonin (5-hydroxytryptamine), a vital mammalian product that inhibits or stimulates smooth muscles and nerves; AT-acetyl-5-methoxytryptamine (melotonin), a constituent of the pineal gland with melanophase-stimulating properties; 5-methoxy- N,AT-dimethyltryptamine, a constituent of the hallucinogenic Virola snuffs; psilocybine, a hallucinogenic found in the mushroom Psilocybe mexicana Heim. The harmala alkaloids, such as harmine and harmaline, are powerful monoamine oxidase inhibitors, previously used in the treatment of Parkinsonism.
Harmine is the active ingredient of the narcotic drug yage. Ellipticine and derivatives show anticancer activity. Several alkaloids of Calycanthus spp. are the products of dimerization or trimerization of simple tryptamine residues, such as folicanthine.
The main indole alkaloid skeletons are derived from tryptamine and a C10 unit. Examples include corynanthine; yohimbine, which has hypotensive and cardiostimulant properties and is used to treat rheumatic disease; ajmaline, which has coronary dilating and antiarrhythmic properties; decarbomethoxydihydrovobasine, which shows vasodilating and hypotensive activities whereas related compounds exhibit antiviral activity; akuammicine; tabersonine; catharanthine; rhynchophylline; vindoline; dihydrovobasine; 10-methoxyibogamine, whose acyl derivatives exhibit analgesic and antiinflammatory activity; and strychnine. This structural diversity has been the source of intense biosynthetic interest.
Physostigmine
Physostigmine, found in the perennial West African woody climber, Physostigma venenosum Balfour, is pharmacologically similar to pilocarpine and is a reversible cholinesterase inhibitor used to treat glaucoma.
The Ergot Alkaloids
The ergot alkaloids are obtained from ergot, the dried sclerotium of the fungus Claviceps purpurea (Fries) Tulsane (Hypocreaceae). Ergot alkaloids are produced by isolation from the crude drug grown in the field, by extraction from saprophytic cultures, and by partial and total synthesis. The ergot alkaloids act pharmacologically to produce peripheral, neurohormonal, and adrenergic blockage, and to produce smooth-muscle contraction as well. The two medicinally important ergot alkaloids are ergotamine and ergonovine.
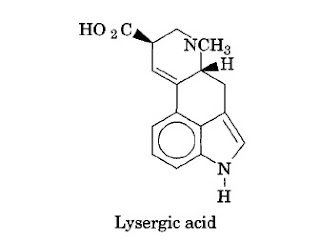 |
Three main groups of ergot alkaloids exist:
- The clavine type, a group of over 20 alkaloids, which is water insoluble and does not give lysergic acid on hydrolysis. This group includes elymoclavine, agroclavine (a potent uterine stimulant), and chanoclavine-I.
- The aqueous lysergic acid derivatives such as ergonovine, which in its maleate salt (or as methyl ergonovine maleate) is the drug of choice to treat postpartum hemorrhage.
- The peptide ergot alkaloids, a group of water insoluble lysergic acid derivatives. This group includes ergotamine, ergocornine, and ergocryptine. Ergotoxine, a mixture of three peptide ergot alkaloids, possesses strong sympatholytic action and is used as a peripheral vasodilator and antihypertensive. Dihydroergotoxine is used for vascular disorders in the aged.
Some ergot alkaloids (eg, 2-bromo-α-ergocryptine) stimulate prolactin release and are being evaluated for treatment of breast cancer.
Catharanthus and Vinca Alkaloids
Catharanthus and Vinca alkaloids, usually discussed together, are quite distinct. The most important alkaloids of the Catharanthus genus are vincaleukoblastine, leurocristine, and leurosine, all antileukemic agents. Vincaleukoblastine and leurocristine are used clinically. The most important alkaloid of Vinca is vincamine, used to treat hypertension, angina, and migraine headaches. Alkaloids of this type produce marked hypotensive effects and curare-like action. The ethers of vincaminol are potent muscle relaxants.
Rauwolfia
Rauwolfia alkaloids include reserpine, the first tranquilizer, rescinnamine, and deserpidine. Reserpine is a sedative and tranquilizer useful in treating hypertension. It is also used as a rodenticide.
Strychnine
Strychnine, from the seeds of many Strychnos species, is a widely known poison (although, in fact, it is only moderately toxic). Pharmacologically, strychnine excites all portions of the CNS; it is a powerful convulsant and death results from asphyxia. It has no therapeutic uses in Western medicine, although its nitrate is used in treating chronic aplastic anemia.
Cinchona Alkaloids
Cinchona alkaloids, derived from the dried stem or root bark of various Cinchona species, include quinine and quinidine. These alkaloids are bitter tasting white crystalline solids, sparingly soluble in water. Quinine is toxic to many bacteria and other unicellular organisms and was the only specific antimalarial remedy until the Second World War. It is a local anesthetic of considerable duration. Quinine is commonly used as the sulfate and dihydrochloride. Quinidine, produced by the isomerization of quinine or found in Cuprea bark, is more effective on cardiac muscle than quinine and is used to prevent or abolish certain cardiac arrhythmias.
Camptothecine
Camptothecine, isolated from the Chinese tree Camptotheca acuminata Decsne, is used to treat cancer in the People's Republic of China.
Histidine-Derived Alkaloids
Histidine-derived alkaloids include pilocarpine and saxitoxin. Pilocarpine stimulates parasympathetic nerve endings and is used to treat glaucoma. The main commercial source of pilocarpine is Pilocarpus microphyllus Stapf., known as Marnham jaborandi. Saxitoxin is an extremely toxic neuromuscular blocking agent found in the so-called coastal red tides of North America.
Monoterpenoid Alkaloids
Monoterpenoid alkaloids include chaksine, a guanidine alkaloid from Cassia lispikula Vahl, which induces respiratory paralysis in mice; β-skytanthine, which is tremorigenic; cantleyine, derived from a monoterpene before loganin; and those derived from secologanin, such as gentianine, which exhibits hypotensive, anti-inflammatory, and muscle-relaxant actions, gentioflavine, gentiatibetine, pedicularine, and actinidine, a potent feline attractant.
Diterpene Alkaloids
Diterpene alkaloids are not of commercial or therapeutic significance, but some have potent pharmacological activity, eg, aconitine and Erythrophleum alkaloids.
Steroidal and Triterpene Alkaloids
Steroidal and triterpene alkaloids are found in the plant families Solanaceae, Liliaceae, Apocynaceae, and Buxaceae. There are four main groups based on the botanical source: the Veratrum, Solanum, Holarrhena and Funtumia, and Buxus alkaloids. The Veratrum alkaloids include jervine, protoveratrine A, and protoveratrine B; the latter two produce pronounced bradycardia and a fall of blood pressure by stimulation of vagal afferents.
The Solanum alkaloids are of interest as potential sources of steroids. Examples of these alkaloids are tomatidine and solanidine. Some Solanum alkaloids exhibit fungistatic activity. Biosynthetically, the alkaloids are derived from acetate and mevalonate.
Table 1. Effects of Natural Food Alkaloids on Humans and Animals
Purine Alkaloids
Purine alkaloids are derivatives of the xanthine nucleus and include caffeine, theophylline, and theobromine, the principal constituents of plants used throughout the world as stimulating beverages. Caffeine has the structure 1,3,7-trimethylxanthine. It is derived from cola, coffee (qv), tea (qv), guarana, and mate.
Theophylline, 1,3-dimethylxanthine, is found in tea. Theobromine, 3,7-dimethylxanthine, is found in cocoa and tea. The xanthine derivatives have pharmacological properties in common: central nervous system (CNS) and respiratory stimulation; skeletal-muscle stimulation; diuresis; cardiac stimulation; and smooth-muscle relaxation.
Caffeine is used to increase CNS activity; it acts on the cortex to produce clear thought and to reduce drowsiness and fatigue. Theophylline is used in smooth-muscle relaxants. Theophylline, as the ethylenediamine salt, is used in preference to caffeine in cardiac edema and in angina pectoris.
Miscellaneous Alkaloids
Coniine is an extremely toxic alkaloid that induces paralysis of the motor nerve endings and is the primary toxic constituent of poison hemlock. It was the first alkaloid to be synthesized. Carpaine, a crystalline macrocyclic alkaloid that induces bradycardia, depresses the CNS and is a potent amoebicide. Alkaloids are found in the poisonous Amanita species of mushrooms, such as α- and β-amanita toxins, ibotenic acid, muscimol, and muscazone. Maytansine and related ansamacrolides are potent antileukemic agents. Surugatoxin, found in the carnivorous gastropod Babylonia japonica, produces a pronounced mydriatic effect, sometimes resulting in death.
lngestion and Human Health
The effects of natural alkaloids on humans and animals vary. This article is concerned mainly with food alkaloids and their clinical effects when ingested intentionally or accidentally. Table 1 summarizes the information.